CONSULTING
SERVICES
Your Partner for Market Entry
SCROLL
Welcome
Japan is the 2nd biggest market in the world for medical devices & services with annual sales of over US$ 35 billion.
Japan offers many opportunities but also many challenges for foreign companies. Without the right strategy and partners, even the best company and product can fail.
MedTech Partners KK is a Tokyo-based consulting firm focusing on helping foreign SMEs, mainly within the medical and life science equipment & services sectors to succeed in Japan.
How We Work
All our work is project based following intensive discussions with the client.
We follow a structured approach with regular update meetings as agreed with the client.
Initial Discussion & Analysis
Understanding your company's product, percieved strengths & weaknesses, pipeline, product life cycle, revenue, regulatory landscape, expectations.
Project work
Deeper market analysis including market development expectations & potential, segmentation, positioning, stakeholder mapping, KOL interviews/surveys & possible clinical trials,
reimbursement situation etc. Client specific requests
Report
Final report delivered to client in accordance with project identifiable, proposal(s), thorough discussions and follow-up as required
Our Expertise
MedTech Partners was founded with the purpose of providing Life Science SMEs with objective, professional consulting services. The founder, Klaus Jacobsen has over 25 years’ experience in managing foreign subsidiaries in various businesses (MedTech & consumables, measuring equipment for industrial applications, automation) in Japan including involvement in the surrounding countries as well.
We are very familiar with the daily operational challenges faced in Japan, whether working through a distributor, a JV, or own subsidiary.
Through its network, MedTech Partners has access to a wide range of specialists from lawyers, tax consultants, regulatory resources, recruiters, and government relation specialists, not to mention its extensive network of medical professionals (KOL), who can assist with clinical opinions, support, and studies when required.
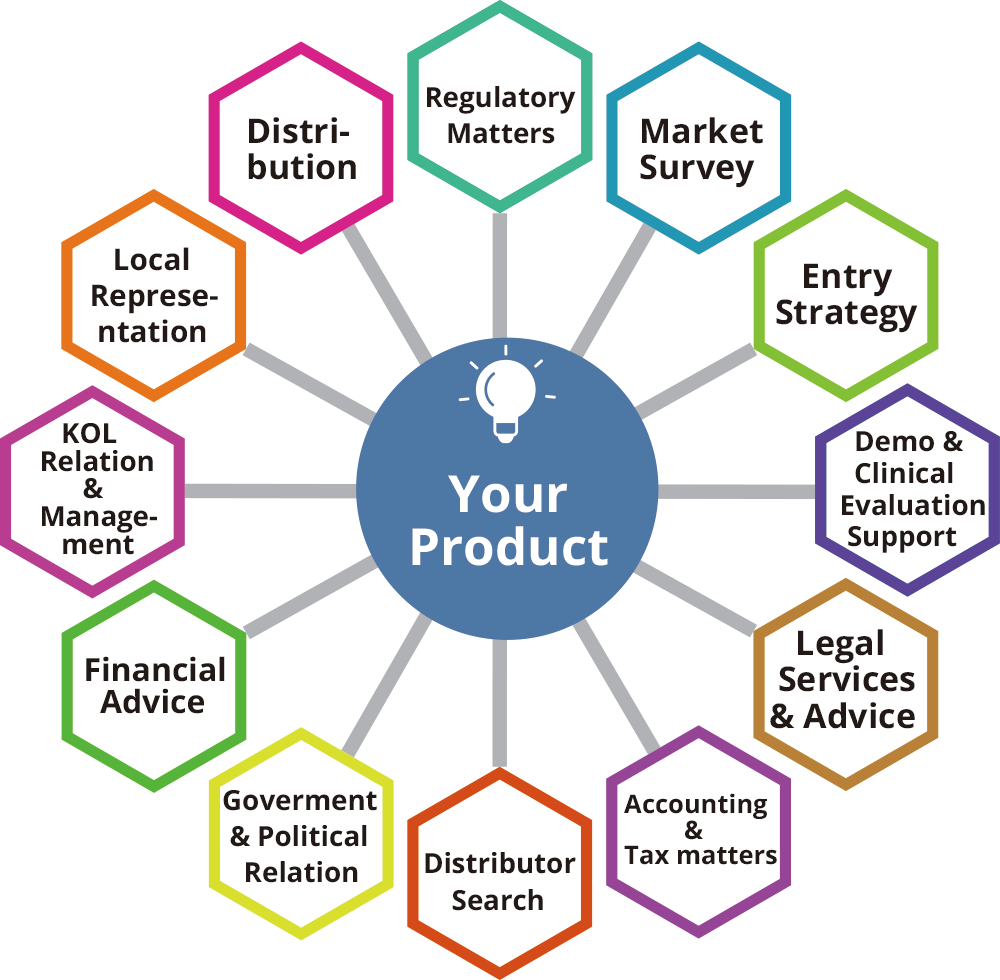
Our approach is based on systematic stakeholder analysis leading to proposals for market entry clearly describing potential, risks, alternatives, and options.
In April 2024, we welcomed Yuki Kato, a longtime colleague, as partner, and began expansion of our consulting services as well as entered into product distribution operations as well.
This enables us to provide an even more comprehensive support.